An extension of your RA team that spans all of the EU & CIS regions.
Timely execution of projects isn’t luck. It takes passion and experience.
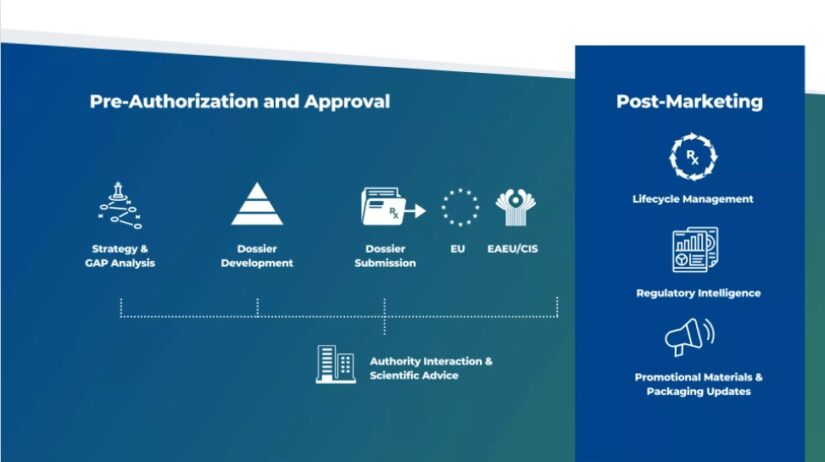
Local regulatory experts for Pharma, Biotech and Medical Device companies across Europe, CIS/EAEU, and MENA regions from dossier development to full life-cycle management.
Riaantech expertise ranges from developing and executing your regulatory strategy to pave the way for Marketing Approval. By combining our in-house regulatory expertise with scientific know-how, we help you to ask the right questions and to keep the end goal in mind during early development. Our focus is on enabling you to navigate the landscape of complicated regulations and compliance hurdles and provide you with a clear strategy for successful commercialization. In addition to complicated regulations and compliance hurdles, we also deliver specific support concerning local needs as they are fundamental to any strategic regulatory decision. As an extension of your team, we cooperate with you every step of the way towards Marketing Authorization.