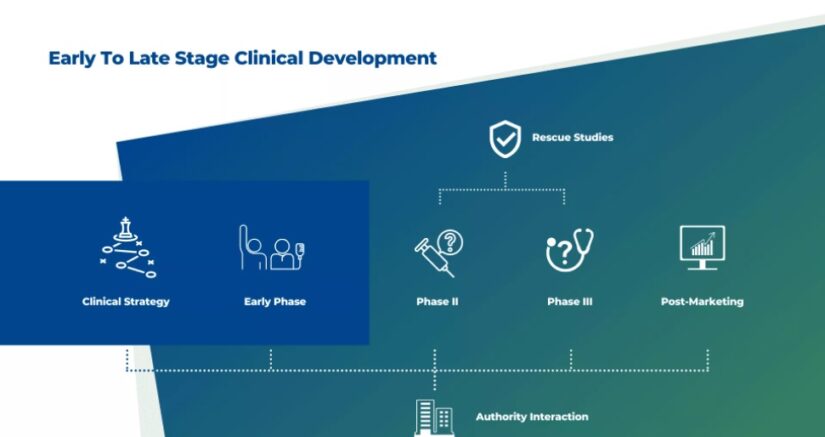
A full-scope partner in clinical development that accelerates your path to market.
Exceeding your timelines isn’t chance. It’s our mindset.
Early clinical development experts with one-stop-shop capabilities in high recruiting countries, accelerating product path to the market.
Our Early Phase Unit BIO1 offers a safe and comfortable environment for patients or healthy volunteers. It provides each customer with a project manager as a principal point of contact who oversees the process from recruitment to clinical study reports. The facility covers all types of early phase clinical studies, including first-in-human and proof-of-concept, single and multiple doses, bioequivalence, bioavailability, drug-drug and drug-food interaction, PK/PD, tQTc studies.